Life cycle assessment of microgreen production: effects of indoor vertical farm management on yield and environmental performance

Case study description
In this study, we utilized LCA, a framework that assesses potential effects on the environment and resources utilized in a production system51. Specifically, we applied LCA to evaluate an IVF installed in the Lisbon region but not yet being commercially exploited. We, therefore, carried out a prospective evaluation, i.e., an LCA used for novel systems or technologies where data are scarce. This study is in accordance with the LCA requirements of ISO Standard 14,044; 2006 where the LCI stage uses mass and energy process-based modelling to build unit processes for plant growth and climatization within the unit and the database ecoinvent 3.8 to determine emissions for background processes48. We used the software OpenLCA version 1.11.0 to calculate unitary emissions for each equipment and material input8,52. Emissions were imported into MATLAB version R2019a53, where the process-based and mass-balance modelling was carried out and final results compiled.
Figure 4 illustrates the procedure for the analysis that will be detailed in the following sections. We used an LCA framework (represented in grey). The LCI (in blue) involved the development of a plant model that estimates yield under a set of operational conditions that define an option space for IVF management, as well as the mass and energy balance of the IVF (in green).
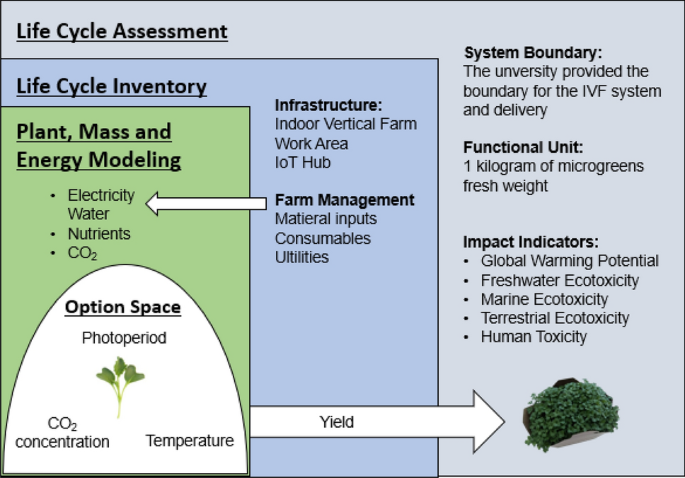
Schematic depiction of the analysis framework; Life cycle assessment where the life cycle inventory was based on plant, mass, and energy process-based modelling with a variable set of parameters explored as an option space.
The IVF modelled here was a prospective building-integrated technology that included a 32 sqm growth chamber with vertical hydroponics and LED lighting system installed inside the technical area of a building on a university campus located in Carcavelos, Lisbon Portugal. Detailed specifications for the infrastructure and combination of ag-tech used in the IVF system (growth chamber, LEDs, and growing systems) were taken from an article by Parkes et al.16. We combined that LCI for the infrastructure (namely the IVF and preparation work area) with adaptations made to estimate the impacts of electricity consumption and additions made to climatization (heating, cooling, and humidity control) for the modelling of dynamic plant growth, as outlined in 5.2.
Goal and scope
The goal of this study aimed to investigate the impact of various operational parameters on microgreen growth and environmental performance within an IVF. The study explored an option space for the management of IVF growing microgreens, where the functional unit was 1 kg of fresh weight kale microgreens supplied locally to the campus. The weekly production was modelled as the average over a 12-month period with a single growth cycle requiring 2 full weeks from seeding to harvest. Life cycle inventory materials were introduced to the process-based plant model as a 1-week cycle of growth, after 7 days of germination. Known as a leafy-green, kale (Brassica oleracea, var. acephala) has published data on the early growth stage, and when grown for 7 to 14 days after germination, the harvested sprouts, and first leaf’s final product is a microgreen with high nutritional value and cultivation density35,38.
Methodologically, we aimed to address the static nature of LCAs regarding the production system’s response to varying conditions inside the IVF, as previous studies consider yield and material inputs as fixed in the LCI54,55. To do so, we use process-based modelling to determine the dynamic relationship between material inputs, environmental conditions, and the response of plant growth inside the IVF. To reduce some complexity, the variables explored relate to those conditions with high impact on energy consumption and under the direct control of IVF management, specifically operating hours of LEDs known as photoperiod, CO2 concentration (associated with fertilization), and air temperature14,57. To investigate the relationship between maintaining ideal growth conditions for crops, yield, and environmental performance, a dynamic LCI was produced in this study. This approach allowed for an exploration of the material input requirements for crop demand and the impact on the final functional unit.
Life cycle inventory
This section describes the fixed variables and the database processes used to estimate their environmental performance (2.2.1), how this LCI was combined with the process-based plant model (2.2.2), and the mass and energy balances of the IVF and subsequent consequences on LCI material inputs (2.2.3). Unlike previous studies, the LCI considered in this study needed variable components for inputs tied to climatization and plant growth for inclusion in the process-based model to explore the option space available for plant growth. Depending on the photoperiod, air temperature, and CO2 concentration inside the IVF, the process-based model estimated the crop yield, water and fertilizer supplied, and the CO2 and heat flow exchanges between the plants and their environment. Based on this internal system dynamics, and through mass and energy balances, the required moisture, CO2, and electricity to maintain air humidity, CO2 concentration, and temperature were calculated. The yield and the required inputs for plant growth and climatization for the conditions assessed could then be included in the inventory.
Inventory for the IVF
Data for IVF infrastructure, equipment and energy, and material inputs for production were sourced primarily from the ecoinvent v3.8 and Agribalyse v3.0.1 datasets48,49,50. The infrastructure of an IVF generally combines three ag-tech: a controlled environment growth chamber, a vertical soil-less growing system, and a lighting system12,16. This LCI includes a LED system for lighting, a vertical hydroponic growing system, and the climate-controlled growth chamber. Those systems require installation materials, LED fixtures, steel structures, trays, climate control equipment, transportation of materials/equipment, and IVF assembly. Key soil-less growing equipment included piping, filters, tanks, nutrient dosing, and water pumps. Additionally, an electrical hub was introduced for LED lighting, climate control, and sensor integration with the associated cables and electronic components. Due to the data requirement of the building-integration in the selected case study an IoT main hub and sensor clusters were all included in the infrastructure. The work area used for operating IVF processes for seeding, harvest, and packaging required a work room with similar inputs as the climate growth chamber. The inventory includes an updated energy mix to improve the accuracy of electricity supply in Portugal, the equipment required for CO2 fertilization, the liquid CO2 dosing via aluminium cylinders, and the humidifiers for relative humidity control (see Supplementary Material, Sections 1.5.1 to 1.5.5). The majority of IVF infrastructure used to produce the functional unit has a 20-year lifetime applied, whereas 10-year lifetime was considered for the LED lights and the growing trays58. All LCI inputs for a cradle-to-gate system boundary were defined, similar to the circular supply proposed for the university campus scenario by Parkes et al.16. Table 1 summarizes the data sources of equipment and materials in the LCI required to execute the growing process for weekly production of kale microgreens for harvest and sale and treatment of the by-product output of compostable biomass.
Processes for IVF operations were replicated in OpenLCA to determine the material inputs consumed to produce the functional unit and the impact of these materials on the environmental performance of the technology. The system boundary incorporates 4 processes described below: Seeding, Growing, Harvesting, and Cleaning. All IVF operations for this study are based on a weekly production cycle. It begins with the seeding process, which produces the seeded tray for germination and growing. The material inputs for the process are based on every kilogram of fresh weight produced and include: 0.07 kg of seeds equivalent to conventional cauliflower seeds in Agribalyse database, 3 kg of coconut fibre with husks for the substrate, plastic sheets, and polyethylene reusable trays. Due to requirements for food safety and sanitization, the IVF requires constant cleaning. We thus included cleaning at the end of the seeding, harvesting, and packaging processes. Cleaning was estimated to take a maximum of 2 h for each functional unit, including soap and water, plus clothes, gloves, and glasses with a 1-year lifetime.
Transferred from the preparation work area, the seeded tray then enters the growing process inside the IVF, where it enters the dark layer without LED light, which is the germination tier of the vertical hydroponic system. Following 7 days, sprouted seeds appear and the trays are moved upwards into the cultivation tier where LED light, water, and nutrient dosing are controlled by interval set points. A flowrate controller was also considered to manage the supply of CO2 to the inside air. For the growing process nutrients are defined as an NPK mix of 15-15-15 supplied by dosing 1 L of water with 15 mL of nutrient mix and the closed loop system produces no waste. The data for consumables supply and delivery are presented in the Supplementary Material, Section 1.5.6.
Operating the IVF requires electricity as a major input for all systems involved in the growing process, from circulating water and nutrients to the energy demand specific for running the climate system and managing indoor environmental conditions. Indoor conditions are changed by controlling temperature (heating and cooling), alternating hours of light from LED (photoperiod), and changing internal atmosphere conditions via humidifiers, air ventilation, and fans. All seeded trays spend a total of 7 days in the cultivation tiers where these conditions were used by the process-based model to predict the effects of changes in these variables on electricity consumption, yield produced and impact indicators.
The energy mix considered in ecoinvent is representative of Portugal in 2018 and was therefore updated with the average production mix available in Portugal between January and August of 2022: 34.1% natural gas, 6.4% fossil CHP, 11.1% hydro, 33.4% wind (with storage), 8.7% bioenergy and 6.6% solar59. Electricity transmission network inputs and emission of sulphur hexafluoride for the voltage conversion process were included. The process considering the different forms of electricity production is presented in Supplementary Material, Section 1.5.1.
After completing 14 days of growing process in the IVF growth chamber, each seeded tray is ready for harvest37. Microgreen shoots and leaves are separated from the roots and substrate in each tray, where the fresh cut kale microgreens are packaged into reusable boxes for delivery on the campus. Leaving the roots and substrate as organic waste, a co-product destined for treatment to create compost for direct use on university gardens with zero emissions, as new compost purchased for campus is avoided. The quantities considered are calculated based on the total fresh weight of microgreens and roots (kg), plus the mass of the substrate as 3 kg per kilogram of kale microgreens.
Process-based model for plant growth
We used the plant growth model by Van Henten30 to estimate the growth of kale microgreens produced inside the prospective IVF technology studied. The model was implemented in MATLAB R2019b53. The original model simulates the growth rate of lettuce (Lactuca sativa L.) with some parameters designed for this species’ full growth cycle, while other parameters were either physical constants or depended on the environmental conditions available for the plant. Though designed as a continuous model, it is applied with discrete intervals based on the farm design31. Figure 5 illustrates the relationship between these IVF conditions and the respective plant growth processes of photorespiration and photosynthesis. The growth rate is a function of the efficiency of conversion from CO2 to mass, and the difference between the CO2 intake during photosynthesis and CO2 released during respiration.
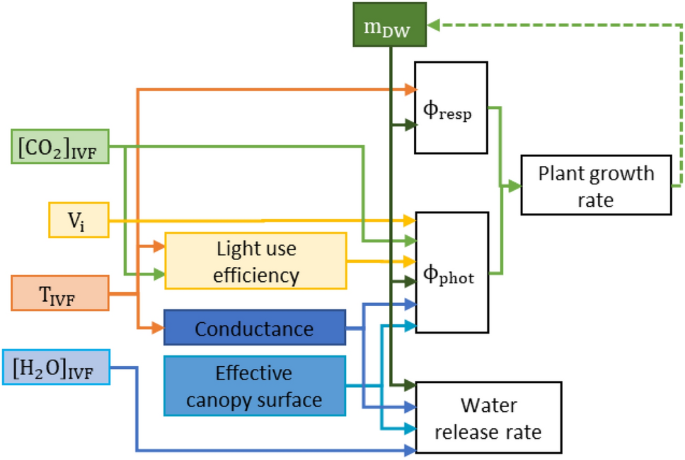
Simplified schematic depiction of the process-based plant model. All variables; CO2 concentration ([CO2]IVF), air temperature, (TIVF), light intensity, (Vi), and humidity ([H2O]IVF), influence directly or indirectly via light use efficiency and conductance, photosynthesis (ϕphot), respiration (ϕresp) and growth rate of plant dry mass, (mDW).
The time step considered in the plant model was 15 min to match IVF management frequency for setting fixed conditions in the farm. Production assumed 7 days of germination, followed by 7 days of growth under lights and then harvesting. Based on this growth cycle the model was changed to use the total photon flux density and photosynthetically active radiation efficiency for lighting (see Supplementary Material, Section 1.2). The original model did not consider water and fertilizer demand as it assumed plants grew in unlimiting conditions. Updates were made to use a unitary transmission coefficient, a dry mass content ratio, calculation of moisture and heat exchange between plants and air; and fertilizer, industrial CO2, and water consumption of plants based on nutrient content. As production is continuous in time, the whole IVF system capacity is 126 trays used in 1 plant growth cycle, but not simultaneously. One-seventh of the trays begin growth every day, ensuring that every day 18 trays are available for harvest, producing a total of 126 trays harvested per week. The individual mass and energy flows were then also translated in time and summed to result in total, continuous flows.
The lettuce-specific parameters were then updated to be representative of kale microgreens. Calibration for kale occurred as a result of selecting a species for production as microgreens and based on the availability of published data for different plant growth conditions on this species. The calibrated parameters were those that played the largest effect in the model30, as presented in the Supplementary Material Section 1.1. Data from two papers were used, namely Ford and Thorne31 who measured kale growth evolution as a function of CO2 concentration and light intensity, and Chowdhury et al.33 who measured growth depending on temperature. As the papers used in calibration did not report growth per unit area, as used in the model, two extra parameters for calibration were considered, which intend to be the best estimates for the area of growth31,33. The calibration took place in MATLAB R2019b53 using the Optimization Toolbox (see Supplementary Material, Section 1.1).
The option space for growth was then determined and used to evaluate the effects on yield of various changes to IVF growth conditions. The option space studied targeted temperature of between 15 to 25 °C in steps of 1 °C, CO2 concentration of 400 to 3300 ppm in steps of 100 ppm, and photoperiod of 8 to 24 h d−1 in steps of 1 h d−1, respectively, resulting in 5610 possible combinations. These values were chosen from the available literature on kale production (Table 2).
Mass and energy balance of the IVF
We calculated mass and energy transfers between the IVF and the building, to determine the heat, humidity, and CO2 exchange of the farm with the outside climate in the technical area. We considered climatization leakage, i.e. loss of CO2, heat, and humidity to the exterior of the IVF due to day-to-day operations19. This includes heat exchanged through the growth chamber walls, released by human workers or functioning equipment, and absorbed by the plants6,61.
Leakage frequently creates an imbalance in calculations due to loss of atmospheric conditions through opening doors or changes in IVF conditions45. The heating, cooling, humidity, and CO2 are added or removed via control of climate systems, which were all calculated by the mass and energy balances of the IVF system. Based on the maximum air flowrate of the climate system, the desired air flowrate and CO2 concentration were calculated. The water mass balance was then calculated to consider water released by human workers and by the plants, which enters the air supply as moisture that was added and/or removed46. Once all mass exchanges were calculated, a heat balance was determined. In this study, the calculations developed in the model consider three main imbalances (found for CO2, moisture, and heat flows due to leakage) that were corrected. Calculations can be found in the Supplementary Material in Section 1.4.
Life cycle impact assessment
For LCIA we explored five main categories of environmental impact: Global Warming Potential (GWP), Freshwater Ecotoxicity (FE), Marine Ecotoxicity (ME), Terrestrial Ecotoxicity (TE), and Human Toxicity (HT)20,22. Calculations of the environmental impacts of each component of the farm were executed in OpenLCA, version 1.11.08,52 making use of the ReCiPe 2016 (H) impact assessment method. Results were then imported to MATLAB, version R2019a53. Knowing the impacts of fixed inputs, such as the infrastructure and seeded trays, and dynamic inputs, such as electricity, water, and nutrient consumption, the overall impacts were calculated as the sum of the impacts of each input for all the option space in a much shorter time, than if all calculations had been performed in OpenLCA.
link





:max_bytes(150000):strip_icc()/EWL-best-no-added-sugar-drinks-to-hydrate-8675270-3x2_preview-c0d05cf54f864461ac848b3bc4b1f12f.jpg)